noble gas configuration of magnesium|Electron Configuration Chart of All Elements (Full Chart) : Bacolod When magnesium loses two electrons to form the Mg 2+ ion, it achieves a stable, noble gas-like configuration similar to neon (Ne). Therefore, the Mg 2+ ion has the electron configuration of [Ne], . Comfort Inn & Suites: Comfort Inn & Suites has exceptional customer service... - See 158 traveler reviews, 51 candid photos, and great deals for Comfort Inn & Suites at Tripadvisor.
PH0 · Noble gas configuration (video)
PH1 · Noble Gas Configuration
PH2 · Magnesium
PH3 · How to Write the Electron Configuration for Magnesium (Mg)
PH4 · How do you write the noble
PH5 · Electron configuration of magnesium
PH6 · Electron Configuration for Magnesium (Mg, Mg2+ ion)
PH7 · Electron Configuration for Magnesium (Mg, Mg2+ ion)
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · Chemistry of Magnesium (Z=12)
PH10 · 5.20: Noble Gas Configuration
Find flights to Manila from ₱18,475. Fly from Prague on Oman Air, China Airlines and more. Search for Manila flights on KAYAK now to find the best deal.
noble gas configuration of magnesium*******Mar 23, 2023
Gas (predicted) Position in Periodic table: Group: 18, Period: 7, Block: p: Category: .
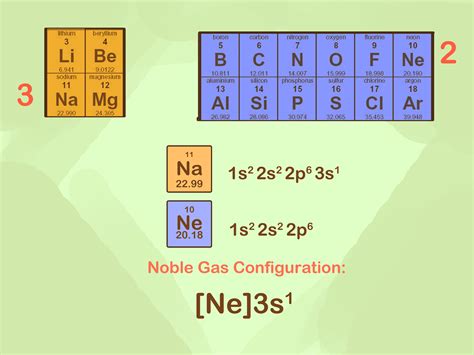
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for . When magnesium loses two electrons to form the Mg 2+ ion, it achieves a stable, noble gas-like configuration similar to neon (Ne). Therefore, the Mg 2+ ion has the electron configuration of [Ne], . How do you write the noble-gas electron configuration for magnesium? | Socratic. Chemistry Electron Configuration Electron Configuration. 1 Answer. .
Electron configuration of magnesium in a simplified way. In a more simplified way, the following configuration is obtained: [Ne] 3s 2. >This summary notation is obtained by .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
General Information. Symbol: Mg. Atomic Number: 12. Atomic/Molar Mass: 24.31. Melting Point: 648.8°C, 921.8K. Boiling Point: 1090°C, 1363K. Density:1.738 g/cc. .
These are our P electrons because they're in P orbitals, and then once we're through our 2p6 electrons, we go to 3s2 and we have two more electrons, so it's 3p2. So that's the .
Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Video: Magnesium Electron Configuration Notation. The configuration notation provides an .These are our P electrons because they're in P orbitals, and then once we're through our 2p6 electrons, we go to 3s2 and we have two more electrons, so it's 3p2. So that's the electron configuration for silicon. Now, we can write it out using noble gas notation. And compare, so, the noble gas immediately preceding silicon, if we go up a row and .

All have an ns 2 np 5 electron configuration, one electron short of a noble gas electron configuration. (Note that the heavier halogens also have filled (n − 1)d 10 subshells, . For example, the last electron added in the .noble gas configuration of magnesiumAll have an ns 2 np 5 electron configuration, one electron short of a noble gas electron configuration. (Note that the heavier halogens also have filled (n − 1)d 10 subshells, . For example, the last electron added in the .Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
Electron Configuration Chart of All Elements (Full Chart) Which element has the noble-gas configuration Ne 3s2? Magnesium is the element that has the noble gas configuration of Ne 3s2. It is a chemical element with a symbol Mg and atomic number 12.ÊThe Noble gas shortcut electron configuration is a way of summarizing the information about the electrons of an atom which shows only the electrons most relevant for understanding the chemistry of the element. Here is a video which discusses how to write the Noble gas shortcut electron configuration for magnesium and lead. Explanation: This video will show you how to write noble gas electron configurations for Mg and Pb. ⚛️Writing Noble Gas Shortcut Electron Configurations - Mr Pauller. Watch on. Hope this helps! Answer link. [Ne] 3s^2 This video will show you how to write noble gas electron configurations for Mg and Pb. In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We’ll also look at why Magnesium forms a 2+ ion and how the electron con.When Magnesium (Mg) forms a cation by losing two valence electrons, it becomes Magnesium cation (Mg2+). The electron configuration of Mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas Neon (Ne). The formation of Magnesium cation (Mg2+) involves the creation of an ionic bond with another element .
The Electron: Crash Course Chemistry #5. Video 3.1.2 3.1. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).
The magnesium ion has an electron configuration like that of which noble gas? Write the electron configuration for the element magnesium. A. 1s^2 2s^2 2p^8 B. 1s^2 2s^2 2p^6 3s^2 C. 1s^2 2s^10 D. 1s^2 2s^2 2p^6 3s^2 3p^2 E. 1s^2 2s^2. 2p^6 3s^1A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1 s2 2 s2 2 p6 part of the configuration. Sodium’s noble gas configuration becomes [Ne]3 s1.A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1s22s22p6 part of the configuration. Sodium's noble gas configuration becomes [Ne] 3s1.Of the ions below, only has a noble gas electron configuration. a) S3- b) O2+ c) I+ d) K- e) Cl-Which noble gas has the same electron configuration as calcium bromide, CaBr2? Which ion does not have a noble gas configuration in its ground state? a. Sc3+ b. Al3+ c. Ga3+ d. As3-Which ion does not have a noble gas configuration in its ground state? A.Noble Gas Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 1 : [Kr] 5s 2 4d 1 . Number of valence electrons: 2 valence electrons that come from the highest shell (n=5). [Kr] 5s 2 4d 1 . Example \(\PageIndex{3}\) Write the electron configuration of mercury (Z = 80), showing all the inner orbitals. What is the nobel gas configuration?Chemistry questions and answers. 7. Write the Noble gas configuration (NGC) of the following ions. Write the symbol of the ion and the charge of the ion. (10 points). a. Sodium ion b. Chloride ion C. Oxide ion d. Magnesium ion e. Sulfide ion.To draw the Lewis dot diagram for magnesium oxide, we need to determine the number of valence electrons for each atom. Magnesium is in group 2 of the periodic table, so it has 2 valence electrons. Oxygen is in group 6, so it has 6 valence electrons. Since we have one magnesium atom and one oxygen atom, the total number of valence electrons is 8.
noble gas configuration of magnesium Electron Configuration Chart of All Elements (Full Chart) The M g2+ cation is isoelectronic with neon, N e, and has a noble gas shorthand notation of. Mg2+:[Ne] Magnesium is located in period 3, group 2 of the periodic table, and has an atomic number equal to 12. This means that a neutral magnesium atom has 12 electrons that surround its nucleus. When magnesium loses two of its electrons, .
Cisco Networking Academy is a skills-to-jobs program shaping the future workforce. Since 1997, we have impacted over 20 million learners in 190 countries.
noble gas configuration of magnesium|Electron Configuration Chart of All Elements (Full Chart)